Two Lots of Blood Pressure Medicine Recalled: Is Yours Affected?
Two types of quinapril and hydrochlorothiazide tablets made by Aurobindo Pharma USA are being recalled because they contain too-high levels of nitrosamines. These compounds are commonly found in water and foods — including meats, dairy products and vegetables in lower levels — but may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time. This is according to the voluntary recall from Aurobindo, which was posted by the US Food and Drug Administration.
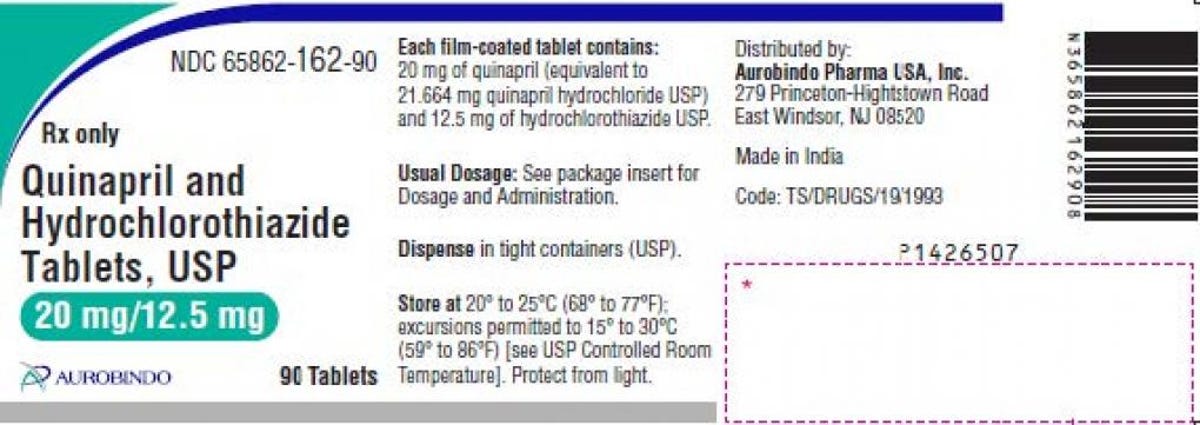
Quinapril and hydrochlorothiazide are used to treat hypertension, or high blood pressure. Two lots of Aurobindo’s 20 mg/12.5 mg are included in the recall, both with expiration dates through January 2023, and the specific lot numbers can be found in the recall announcement on Oct. 25. The tablets are pink-colored and round, with “D” printed on one side and “19” on the other side.
If you have this medication, you should talk with your doctor before you stop taking it, the notice posted by the FDA said. Untreated high blood pressure can raise the risk of stroke, heart attack and other serious health issues or medical emergencies, so you should discuss the risks and benefits of finding another medication.
The hypertension medication label provided in the recall.
FDA
To date, Aurobindo said, it hasn’t received any reports of adverse events related to the recall. Consumers with medical questions about the recall can contact the company at 866-850-2876 (option 2) or email Aurobindo at pvg@aurobindousa.com. For questions about returns, consumers can call 888-504-2014.
Other blood pressure medications under different brands have been recalled this year over the same concern about excessively high nitrosamine levels. As The New York Times reported, the “wave” of recalls is likely due to an increase in testing for things like nitrosamines. Many drug or cosmetic recalls over potential contamination or impurities aren’t meant to cause alarm or to say consumers will be immediately harmed when using a recalled product. Instead, the concern is typically over frequent use over a long period of time.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.
No Byline Policy
Editorial Guidelines
Corrections Policy
Source