Florida woman files lawsuit after infected drops allegedly led to loss of her eye
A Florida woman has filed a lawsuit against the maker of eye drops that were recalled after they were found to be contaminated and allegedly left her legally blind.
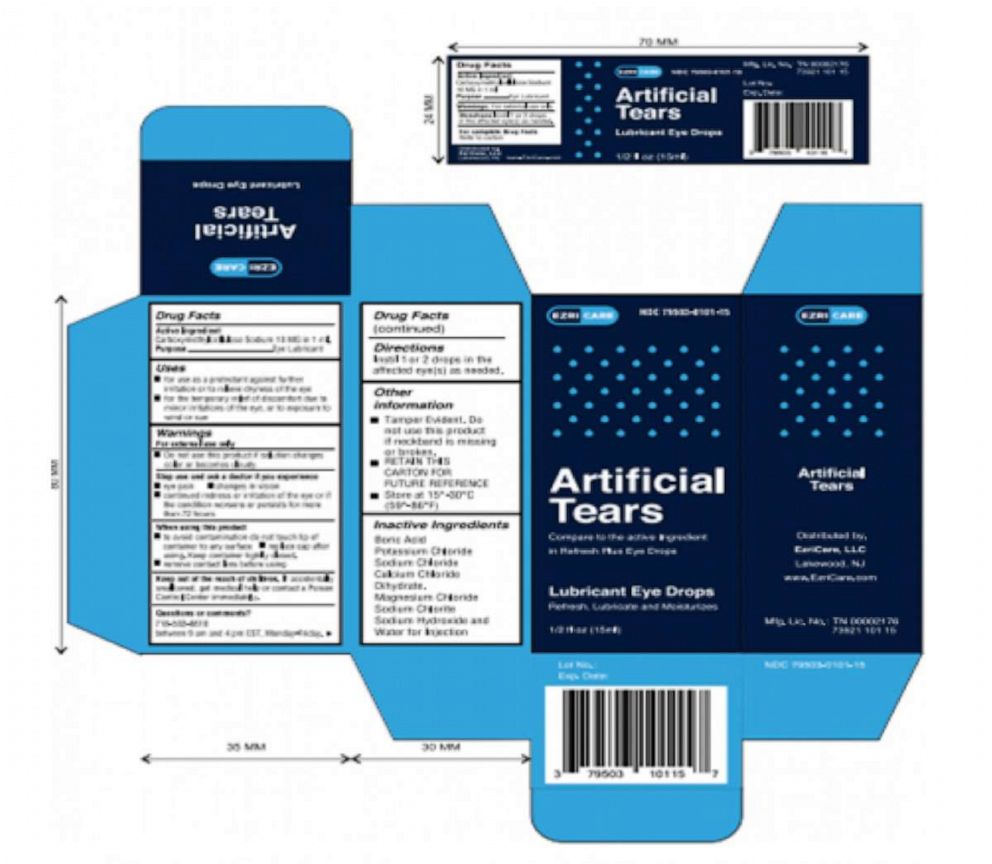
Clara Oliva, 68, of Miami, is seeking damages from Global Pharma Healthcare, which manufactures EzriCare Artificial Tears, as well as the medical center that prescribed her the eye drops and her insurer.
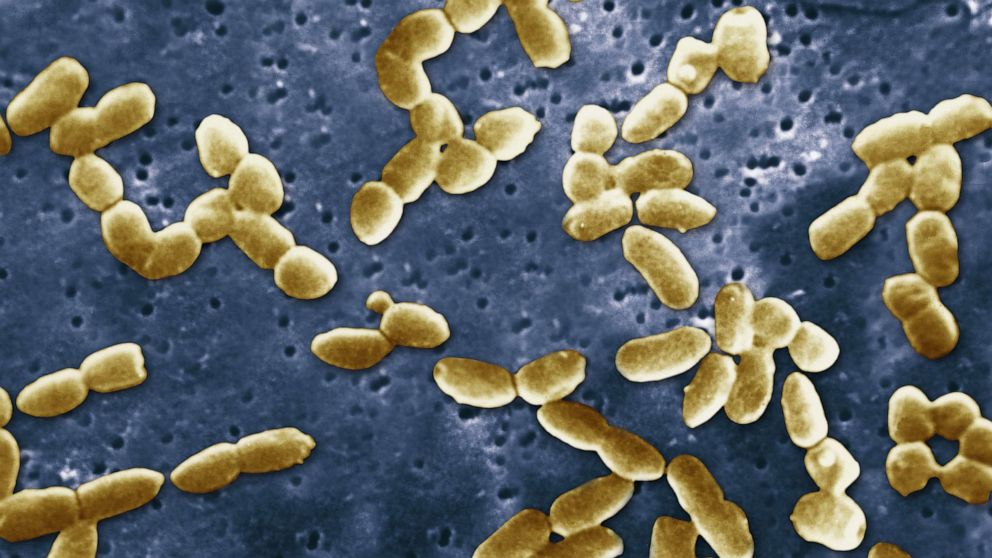
Oliva is one of at least 68 Americans who have used eye drops later found to be contaminated with a drug-resistant form of Pseudomonas aeruginosa, an aggressive bacterium, and developed infections, according to the Centers for Disease Control and Prevention.
The CDC’s latest update published Tuesday, stated three people have died during the course of the outbreak, eight reported vision loss and four had their eyeballs surgically removed.
Eyedrops allegedly cause infections, nationwide outbreak
According to the lawsuit, reviewed by ABC News, Oliva had taken eye drops for years to address her dry eyes caused by contact lenses.
In May 2022, she was told the eye drops authorized by her insurer had changed and she was prescribed EzriCare Artificial Tears by her regular clinic, Leon Medical Centers in Miami, according to the lawsuit.
Global Pharma Healthcare issued a voluntary nationwide recall of Artificial Tears Lubricant Eye Drops due to possible contamination.
FDA
Oliva did not have any issues until early August 2022, when her right eye suddenly became red, swollen and “abnormally watery,” according to her lawsuit.
An ophthalmologist at Leon Medical Centers examined Oliva and allegedly told her she likely had a corneal scratch, which is an abrasion on the cornea, or the clear, protective covering of the front of the eye.
She was given a host of medications, which Oliva said did not improve her eye and the infection worsened. A few days later, she said she visited the emergency room at Bascom Palmer Eye Institute in Miami.
“The pain that I felt was like shards of glass that would move within the eye and it was extraordinarily painful,” Oliva told ABC News through her attorney, Natasha Cortes.
The lawsuit said a biopsy of her eye was performed. At first, doctors believed she had a fungal infection, but they came to discover she actually had an infection caused by P. aeruginosa.
Pseudomonas are a type of bacteria found in the environment like in water or soil, with P. aeruginosa being the most common to cause infections in humans. It usually causes outbreaks in hospitals and nursing homes.
Oliva loses her eye
Oliva said her doctors thought the cause of the infection had been linked to her contact lenses, but she disagreed.
“I had been using contact lenses for 30 years without any issue and I was always meticulous in my care,” she said. “Eventually, they told me that they just didn’t know what it was that had occurred, and I was never satisfied with those answers.”
Surgeons attempted to remove the damaged portion of Oliva’s cornea and replace it with donor tissue, but the procedure was canceled while in progress because they found more extensive damage and the cornea couldn’t be safely removed, according to the lawsuit.
Doctors felt they had no other choice but to remove her eye, Oliva said. She said she begged doctors to try to save her eye, but her medical team was worried if they didn’t act soon, the drug-resistant bacteria could spread.
Pseudomonas Aeruginosa is shown under a scanning electron microscope.
UIG via Getty Images, FILE
“On September 1, 2022, Mrs. Oliva’s right eye was surgically removed and replaced with a plastic implant,” the lawsuit reads. “Given her decreased visual acuity of 20/200 in her remaining left eye, Mrs. Oliva is now legally blind.”
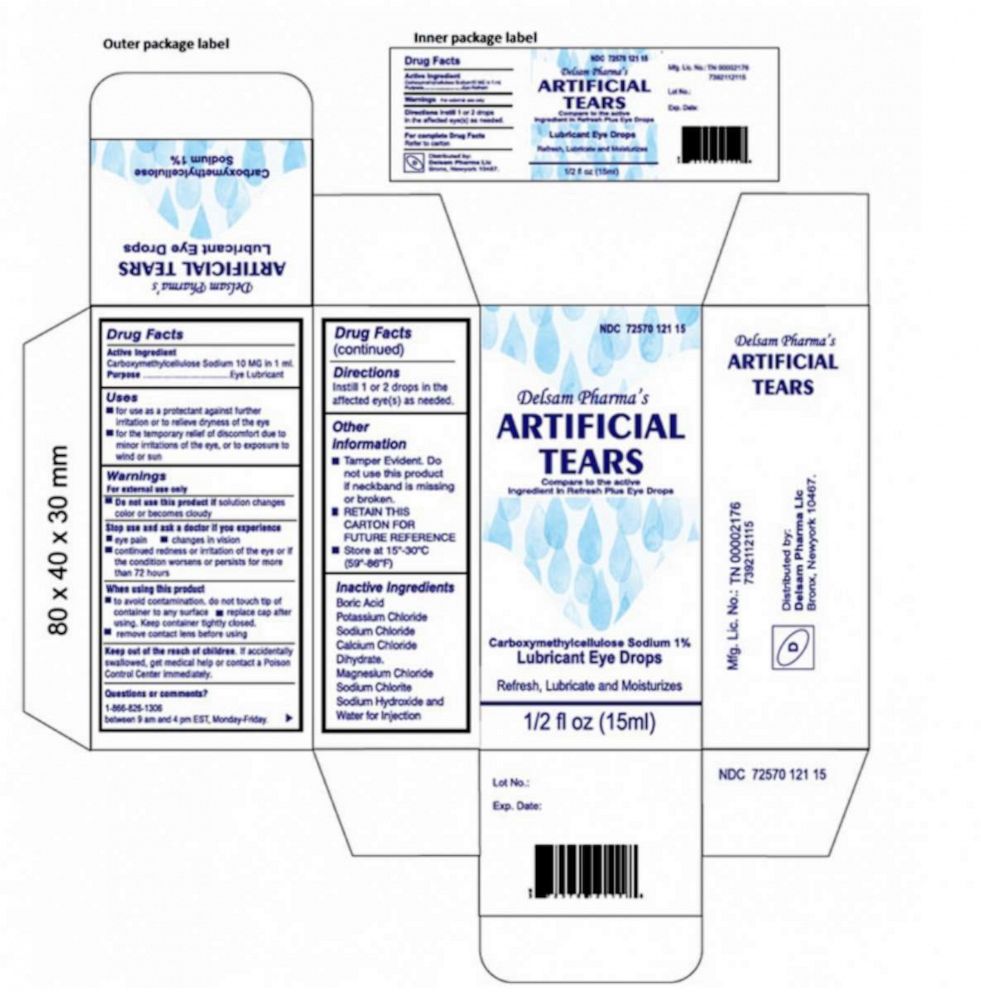
Oliva kept using EzriCare drops on her left eye until January, when Leon Medical Centers, according to the lawsuit, called and told her to stop using the drops because they had been recalled, but she wasn’t told why.
Last month, the Food and Drug Administration issued a warning, backed by the CDC, urging health care personnel and the public not to buy EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to potential bacterial contamination.
Global Pharma Healthcare issued a voluntary recall of both products, notifying distributors and advising wholesalers, retailers and customers who have the products to stop usage.
The company did not respond to ABC News’ request for comment. However, in a statement in early February, EzriCare said it first became aware of the ongoing CDC investigation in January and was cooperating with officials.
“As of today, we are not aware of any testing that definitively links the Pseudomonas aeruginosa outbreak to EzriCare Artificial Tears,” the statement read. “Nonetheless, we immediately took action to stop any further distribution or sale of EzriCare Artificial Tears. To the greatest extent possible, we have been contacting customers to advise them against continued use of the product. We also immediately reached out to both CDC and FDA and indicated our willingness to cooperate with any requests they may have of us.”
Oliva says she first found out the reason behind the recall while watching a TV news report with her family.
“I had conflicting emotions,” she said. “I knew now what had caused all my symptoms and the ordeal that I had gone through but, at the same time, I felt horrible and horrific that these companies could allow this to happen to me and others.”
”There has to be justice”
These are dozens of counts alleged in the lawsuit including negligence and strict liability, which is when a defendant is liable for committing an action regardless of intent.
Oliva is seeking to recoup the cost of her medical care, as well as other damages, but the lawsuit did not state a specific figure.
Oliva said her life has been impacted “1,000%” since the loss of her right eye.
She has had to relearn how to walk, her depth perception has greatly diminished, and she can only drive her car short distances, according to the lawsuit. Additionally, she is under psychiatric and psychological treatment for depression.
Global Pharma Healthcare issued a voluntary nationwide recall of Artificial Tears Lubricant Eye Drops due to possible contamination.
FDA
“They don’t realize the damages that they have caused to me and to others who have been damaged,” she said. “Due to their irresponsibility, their negligence and their incompetence they’ve caused not only deaths, but they’ve affected my life and its entirety and my ability to be independent and live a normal life and there has to be justice.”
In a statement to ABC News, Leon Medical Centers said it “empathizes” with Oliva’s condition.
“Upon learning of the advisory from the CDC in January, Leon Medical Centers immediately began proactively reaching out to its patients that may have received Ezri Care artificial tears and instructed them to discontinue the use of the product and return or discard it,” the statement said. “We will continue cooperating with and monitoring the public health authorities regarding their multistate inquiry into this matter and will provide additional information to our patients as necessary.”
The insurer, HealthSpring of Florida, did not reply to ABC News’ request for comment.
No Byline Policy
Editorial Guidelines
Corrections Policy
Source