Pharma Sector Should Push Manufacturing Patents for Biologics
Although manufacturing methods are important to the process of producing biologics, pioneering companies have favored trade secrets. This reluctance to patent methods of manufacturing lies in the difficulty of discovering infringers, balanced with a desire not to disclose valuable methods.
It’s time to reassess this strategy—based on patents that are showing up in litigation from reference product sponsors to block biosimilar competition. The Biologics Price Competition and Innovation Act is working as designed to provide discovery of a biosimilar manufacturer’s method of manufacturing. As a result, the vast majority of patents asserted in BPCIA litigation to block biosimilar competition are manufacturing patents.
But an analysis of biologics companies’ patent prosecution reveals that they haven’t increased their prosecution of method of manufacturing patents to reflect the greater importance of these patents in BPCIA litigation.
Method Relevance
The very nature of a biologic often depends upon its manufacturing process. It’s easiest for a biosimilar manufacturer to stay as close as possible to the manufacturing process used to make the reference product. This unique importance of methods of manufacturing was recognized in the BPCIA. In contrast to Hatch-Waxman litigation for small molecule drugs, BPCIA litigation can include method of manufacturing patents.
Disclosure Required
A biologics license application (aBLA) must contain a “full description of manufacturing methods,” according to the BPCIA. The first step of the patent dance, or BPCIA framework, requires the biosimilar applicant to provide its aBLA and other information describing its manufacturing process to the reference product sponsor.
While the US Supreme Court held in Sandoz v. Amgen that this disclosure can’t be enforced by injunction, failing to do so allows the reference product sponsor to skip the patent dance and bring suit “with respect to any patent that could have been” listed during the patent dance.
The Federal Circuit suggested in Amgen v. Hospira that if the biosimilar applicant hasn’t disclosed its aBLA and/or manufacturing information, and the reference product sponsor is uncertain of infringement, the reference product sponsor could list a manufacturing patent it reasonably believed was infringed during the patent process.
The biosimilar manufacturer would then be obligated to provide a detailed factual noninfringement response. Failing to do so would provide the reference product sponsor with the basis for an infringement suit.
Production From Biosimilar Manufacturers
An analysis of the pleadings in all 37 BPCIA litigations shows that biosimilar manufacturers are producing their manufacturing information, primarily in the form of their aBLA’s “full description of manufacturing methods”:
- In 71% of cases—and all recent cases—the biosimilar manufacturer produced its unredacted aBLA
- In no cases since Sandoz v. Amgen (and only 5 overall) did the biosimilar manufacturer initially refuse to produce its aBLA
- By far the most common response by biosimilar manufacturers has been to produce the aBLA but no additional manufacturing information (57%), versus aBLA and additional manufacturing information (14%), redacted aBLA (16%), or no aBLA (14%)
Thus, reference product sponsors can be assured they will obtain discovery of a biosimilar manufacturer’s manufacturing methods.
Patent Role in Litigation
An analysis of all 172 patents asserted in the 37 BPCIA litigations shows the overwhelming importance of method of manufacturing patents:
- Over 75% of patents asserted across all BPCIA litigations have been manufacturing patents
- At least half of asserted patents were manufacturing patents in 82% of all BPCIA litigations
- At least two-thirds of asserted patents were manufacturing patents in 59% of all BPCIA litigations
A product-by-product analysis for the 14 reference products involved in BPCIA litigation emphasizes the overwhelming importance of method of manufacturing patents:
- Across all products, an average of 65% of asserted patents were method of manufacturing patents
- At least half of the asserted patents were manufacturing patents for 10 of 14 (71%) reference products
- At least two-thirds of the asserted patents were manufacturing patents for 8 of 14 (57%) reference products
This dominance of method of manufacturing patents in BPCIA litigation further highlights that biosimilar manufacturers are indeed producing their manufacturing information.
Lack of Manufacturing Patents
These data across litigations and products show that the most valuable patents to prevent biosimilar competition are method of manufacturing patents. However, most companies haven’t adjusted their patent prosecution strategies to prevent biosimilar competition accordingly.
A Minesoft Origin analysis of the percent of published patent applications with a method of manufacturing first claim out of all applications examined by the biologics art units indicates no current change from the pre-BPCIA level of about 4%.
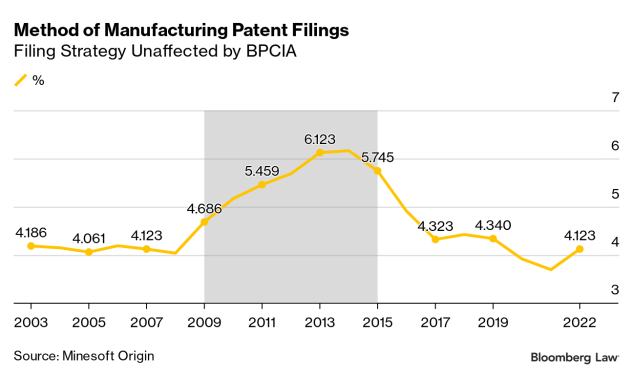
The uptick in 2008 can’t be due to the BPCIA, passed in 2010. More likely, the search picked up an unrelated increase in diagnostic method patent applications as companies tried different strategies to address the increased scrutiny the US Patent Office and courts were giving to this area.
Since method of manufacturing patents are really important in BPCIA litigation, biologics developers should emphasize these patents more, consider new filings they may be holding as trade secrets, and review existing portfolios to see if they support method of manufacturing claims that could block biosimilar competition. Patent prosecution strategy should reflect the realities of BPCIA litigation.
Biosimilar manufacturers are producing their aBLA’s—including manufacturing information—as envisioned by the BPCIA. And most patents asserted in BPCIA litigation are method of manufacturing patents.
Companies’ patent prosecution doesn’t reflect these facts, favoring trade secrets due to old fears about the difficulty of discovering competitor’s methods of manufacturing. Considering BPCIA litigation, companies should reevaluate their patent prosecution strategy for existing and new filings to put greater emphasis on method of manufacturing patents.
This article does not necessarily reflect the opinion of Bloomberg Industry Group, Inc., the publisher of Bloomberg Law and Bloomberg Tax, or its owners.
Author Information
Michael Siekman is a partner in McDermott Will & Emery, where he focuses his practice on intellectual property matters in the life sciences industry, including patent prosecution, licensing, post-grant proceedings and litigation.
Brennen Baylor is an associate in McDermott Will & Emery, where he focuses his practice on intellectual property matters in the life sciences industry, focusing on patent prosecution, patent and trademark strategy and IP litigation.
Write for Us: Author Guidelines
No Byline Policy
Editorial Guidelines
Corrections Policy
Source
